Keywords: Chimeric Antigen Receptor T cell, Pharmacoeconomic, Pediatric, Clinical Trials
With the success of Chimeric Antigen Receptor (CAR) T-cell therapy in the pediatric population, new clinical trials have surged worldwide. To date, no previous study has analyzed the effect of socioeconomic factors on the development of clinical trials for CAR T-cells in the pediatric population. In recent years new countries and emerging economies have contributed significantly to the development of CAR T-cell clinical trials. A better understanding of the socioeconomic factors that influenced these trends will facilitate the future development of this cellular therapy field.
To assess the impact of socioeconomic factors related to the development of clinical trials on CAR T-cell therapies in the pediatric population, we analyzed data entries from clinical trials registered to the Clinicaltrials.gov database. To query the information, we used the following keywords “(leukemia OR cancer OR lymphoma OR malignancies OR tumor OR solid tumor) AND (CAR OR chimeric antigen receptor OR CAR T-cell OR adoptive immunotherapy OR immune effector OR cellular therapy) AND (Child)”. In addition, we used different filters that allowed the selection of studies that focused on patients 18 years of age and younger. Data corresponding to entries with a data cut-off of July-2023 were included. In addition, we use the World Bank country's macroeconomic database (January 1 st, 2009 to December 31 st, 2022).
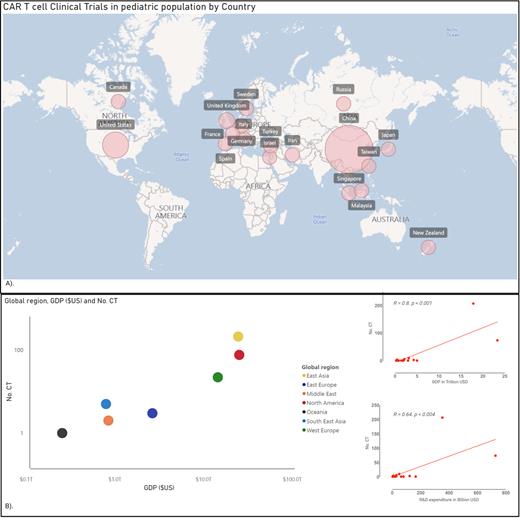
We found 4,297 results in the Clinicaltrials.gov platform; we excluded 3,978 clinical trials that did not involve the pediatric population (≤18 years) or CAR T-cell intervention. There were 319 clinical trials included, sponsored in 19 countries, as shown in Figure A. Of those studies, 207 (64.9%) were conducted in China, 74 (23.2%) in the U.S., and 21 (6.5%) in Europe. Not surprisingly, the number of clinical trials is strictly proportional to each country's Gross Domestic Product (GDP) and Research and Development (R&D) expenditure, as shown in Figure B. Pearsons' coefficient between GDP and the number of trials registered per country is 0.8 (p-value < 0.001); similarly, Pearsons's coefficient between R&D expenditure and the number of problems reported per country is 0.64 (p-value < 0.004). The United States and China are developing 88% of the Clinical trials, and 96% of the clinical trials occur in countries ranked in the top 10 highest GDP.
Our database analysis shows a notable disparity in the countries leading cutting-edge research in CAR T-cell therapy for pediatric oncology. The primary locations for these clinical studies are countries ranked in the top 10 in terms of GDP, with China and the USA being the main contributors. Access to clinical trials plays a crucial role in improving cancer patients' outcomes, and this disparity in the development of cellular therapies is likely to impact children from middle and low-GDP countries significantly. Urgent strategies are required to address this issue and ensure more equitable access to advanced technologies and clinical trials in cellular therapy for pediatric cancers. These strategies should become a high priority for national and international health departments and organizations in the coming years. By promoting more comprehensive clinical trial access and collaboration, we can work towards reducing these disparities and providing better treatment options for all children facing pediatric cancer.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal